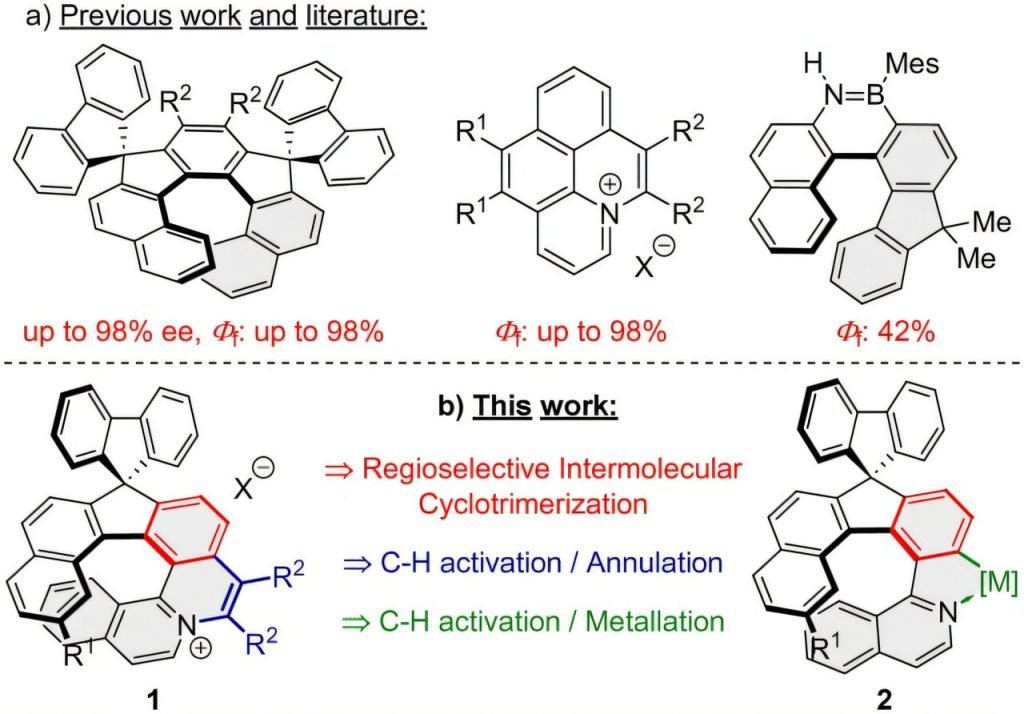
A research team has successfully synthesized a new class of helical quinolizinium salts exhibiting exceptionally strong fluorescence in the orange-to-red light region (606–682 nm).
The work, published in the journal Chemical Communications, combines cutting-edge rhodium-catalyzed [2+2+2] cyclotrimerization and C–H activation strategies to efficiently build complex helical molecular architectures with remarkable optical properties.
In the first stage, appropriately substituted diynes were subjected to Rh-catalyzed cyclotrimerization with trimethylsilylethyne, yielding 1-arylisoquinolines in isolated yields of up to 61%. These intermediates then underwent C–H activation and annulation with various aryl and alkyl disubstituted alkynes, forming [7]-helical quinolizinium salts in yields reaching 93%.
The team also explored enantioselective C–H activation, achieving up to 62% enantiomeric excess (ee), marking a promising step toward chiral control in helical aromatic systems. In addition, boron and platinum complexes derived from the 1-arylisoquinolines were successfully prepared, further expanding the material’s photophysical diversity.
All synthesized compounds display strong fluorescence quantum yields (ΦF = 28–99%), making them attractive candidates for applications in organic light-emitting diodes (OLEDs), sensors, and photonic materials.
More information:
Timothée Cadart et al, Synthesis of highly fluorescent helical quinolizinium salts by a Rh-catalyzed cyclotrimerization/C–H activation sequence, Chemical Communications (2025). DOI: 10.1039/d4cc06512c
Provided by
Charles University
Citation:
Researchers develop highly fluorescent helical quinolizinium salts via rhodium-catalyzed synthesis (2025, October 28)
retrieved 29 October 2025
from https://phys.org/news/2025-10-highly-fluorescent-helical-quinolizinium-salts.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.